On July 29, 2025, the team led by Professor Bing Zhou from Shenzhen University of Advanced Technology (SUAT) published a significant study titled “SLC39A13 Regulates Heart Function via Mitochondrial Iron Homeostasis Maintenance” in the top-tier cardiovascular journal Circulation Research (CAS Q1, latest IF 16.2). This study is the first to reveal a novel mechanism by which the iron transporter SLC39A13 (also known as ZIP13), a key endoplasmic reticulum (ER)/Golgi-resident iron transporter, regulates heart function by maintaining mitochondrial iron homeostasis. It opens one critical pathway for understanding the mechanisms of heart failure and developing new therapeutic approaches.
01 Core Challenges in Cardiac Energy Metabolism
The heart, as the body’s “perpetual engine”, relies on a continuous energy supply to sustain its robust pumping function. In cardiomyocytes, mitochondria serve as the central hub for energy production, with iron playing a pivotal role. Recent studies indicate that mitochondrial iron homeostasis is essential for maintaining heart function. Iron is not only a critical component of heme and iron-sulfur clusters but also a key mediator of electron transfer in the mitochondrial respiratory chain. However, some key iron questions remain in cardiovascular research: How is intracellular iron metabolism homeostasis maintained? Is there a complex and precise regulation of mitochondrial iron levels? And how does the disruption of this homeostasis impact heart function? Investigating the mechanisms of iron metabolism in cardiomyocytes and identifying key molecules regulating mitochondrial iron homeostasis are crucial for understanding the pathogenesis of heart disease and developing novel therapies.
02 Paradigm-Shifting Insights into ZIP13 Function
Previously classified as a zinc transporter, ZIP13 was identified by Zhou’s team through studies in Drosophila and mammalian models as an ER/Golgi-resident iron transporter1, 2, responsible for transporting cytosolic iron to the ER/Golgi for use in the secretory pathway. In this study, the team developed a cardiomyocyte-specific Zip13 knockout mouse model (Zip13-CKO), which exhibited a sharp decline in survival rate after three months of age, accompanied by hallmark heart failure phenotypes such as reduced ejection fraction, elevated serum cTn-I/BNP levels, and cardiac chamber enlargement. Using a tamoxifen-inducible Zip13 knockout model (Zip13-iKO) in adult mice, the team further ruled out developmental interference, confirming ZIP13’s sustained role in maintaining adult heart function.
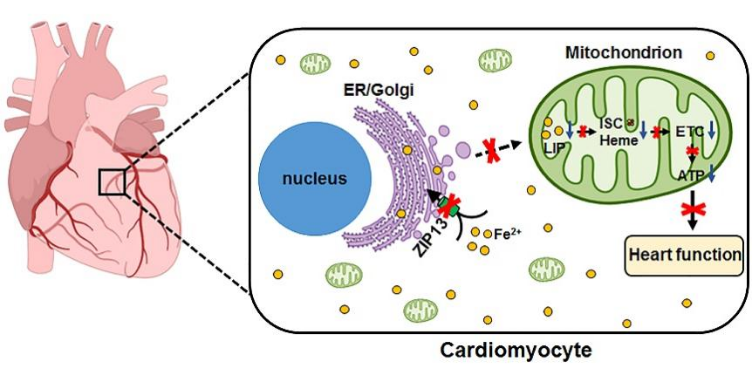
Figure 1: Regulation of Intracellular Iron Homeostasis by the ER/Golgi Iron Transporter SLC39A13/ZIP13
SLC39A13/ZIP13, as an ER/Golgi-resident iron transporter, not only directly regulates iron levels in these compartments but also indirectly influences iron homeostasis in the cytosol, lysosomes, and mitochondria. Its functional loss leads to mitochondrial dysfunction, resulting in impaired cardiac contractility.
03 Core Mechanism of Heart Failure Triggered by Mitochondrial Iron Depletion
In-depth mechanistic studies revealed that ZIP13 deficiency leads to cytosolic iron overload and mitochondrial iron depletion, accompanied by impaired iron-sulfur (Fe-S) cluster biosynthesis. This Fe-S synthesis defect results in reduced activity of Fe-S-dependent enzymes (e.g., aconitase and respiratory chain complexes I/II), decreased ATP production, lowered mitochondrial membrane potential, and structural mitochondrial damage. Notably, iron supplementation (FAC) or overexpression of the mitochondrial iron transporter MFRN1 effectively restored mitochondrial iron levels, respiratory chain function, and cardiomyocyte contractility.
04 Discovery of the Inter-Organellar Iron Transport Network
The observation of ZIP13 on mitochondrial iron homeostasis indicates the existence of an inter-organellar iron transport network from the ER/Golgi to mitochondria. It is not certain, however, how iron traverses along this newly identified pathway. Is it via a direct mitochondria and ER/Golgi touch, or indirectly through lysosome-mitochondrion contact? This question awaits future characterization.
To further clarify the specific role of ZIP13 in cardiac iron homeostasis, the research team also compared its function with that of the key iron exporter FPN1. The findings revealed:
1.Fpn1 Single Knockout (Fpn1-CKO): Primarily led to cytosolic iron accumulation, with no significant changes in mitochondrial iron levels, and resulted in pathological myocardial fibrosis.
2.Zip13 Single Knockout (Zip13-CKO): Characteristically caused mitochondrial iron depletion and contractile dysfunction, with no evident fibrosis.
3.Double Knockout (Zip13&Fpn1-CKO): ZIP13 deficiency exacerbated cytosolic iron overload caused by FPN1 deficiency, leading to significantly greater cardiac functional impairment compared to single knockouts.
These results indicate that ZIP13 and FPN1 maintain heart function through distinct but partially overlapping mechanisms: FPN1 sustains cytosolic iron homeostasis by exporting iron, while ZIP13 maintains cardiac function by balancing iron distribution between the cytosol and organelles, particularly mitochondria.
These studies establish ZIP13 as a critical regulator of mitochondrial iron homeostasis for the first time and highlights the pivotal role of the ER/Golgi-mitochondria iron transport pathway in cardiac energy metabolism. This discovery not only expands the understanding of the cardiomyocyte iron metabolism network but also provides a new theoretical framework for the pathogenesis of heart diseases, particularly heart failure, from a metabolic perspective. Notably, the study found significantly reduced ZIP13 expression in cardiac tissue from patients with ischemic heart disease and dilated cardiomyopathy, suggesting its potential as a novel biomarker and therapeutic target for cardiovascular diseases. Future targeted interventions focusing on ZIP13 and its regulated mitochondrial iron homeostasis pathway (e.g., precise iron homeostasis regulation or mitochondrial iron delivery technologies) hold promise for breakthrough treatments for heart failure and related conditions.
The first author of the paper is Assistant Researcher Li Huihui from the Shenzhen Institutes of Advanced Technology (SIAT), Chinese Academy of Sciences, specializing in iron metabolism, particularly intracellular iron homeostasis. The corresponding author is Professor Bing Zhou from Shenzhen University of Advanced Technology (SUAT), who has long focused on metal ion transport and metabolism, achieving a series of original contributions in the mechanisms of metal metabolism regulation and their roles in diseases.
For further dissemination and exchange of cutting-edge research, researchers in related fields are welcome to discuss and collaborate. For more details, please contact the corresponding author or refer to the original publication.
1. Li H, Cui Y, Hu Y, Zhao M, Li K, Pang X, et al. Mammalian slc39a13 promotes er/golgi iron transport and iron homeostasis in multiple compartments. Nat Commun. 2024;15:10838
2. Xiao GR, Wan ZH, Fan QW, Tang XN, Zhou B. The metal transporter zip13 supplies iron into the secretory pathway in drosophila melanogaster. Elife. 2014;3
Paper Link: https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.125.326201
