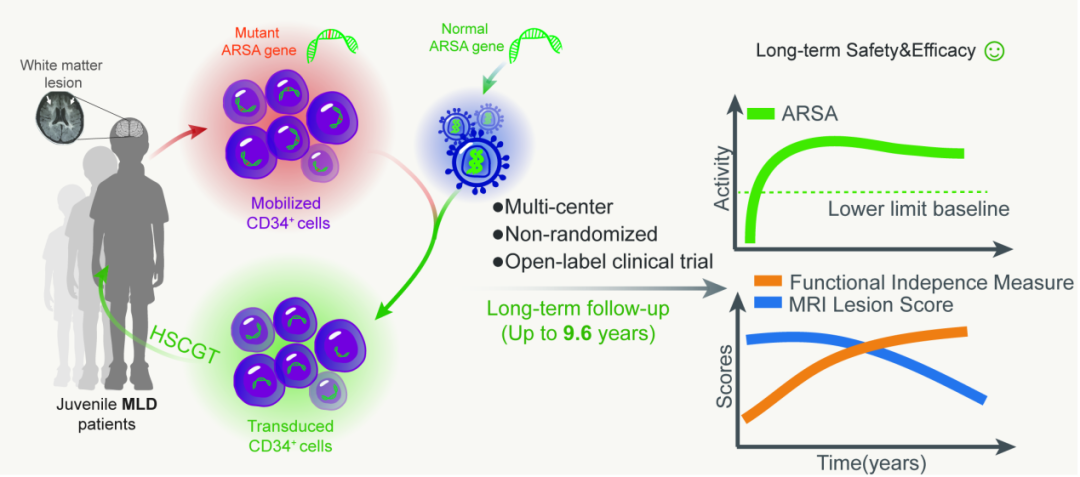
Metachromatic leukodystrophy (MLD) is a rare genetic disorder caused by a deficiency of the enzyme arylsulfatase A (ARSA), resulting in pathological accumulation of sulfatides in the nervous system. This accumulation causes demyelination, neuroinflammation, and neurodegeneration, manifesting as rapidly progressive neurodegenerative symptoms. Currently, there is no curative treatment for MLD. Available therapeutic strategies, including ARSA enzyme replacement therapy, adeno-associated virus(AAV)-mediated gene therapy, and allogeneic hematopoietic stem cell transplantation (HSCT), demonstrate limited clinical efficacy. In April 2024, the FDA approved the clinical use of Lenmeldy™(atidarsagene autotemcel), a hematopoietic stem cell gene therapy (HSCGT) developed by Orchard Therapeutics.. However, this therapy is limited to treating presymptomatic late-infantile MLD and presymptomatic or early symptomatic juvenile-onset MLD patients, restricting its benefit to a narrow patient population. Due to limited awareness of MLD, most MLD cases are identified only after symptom onset. Notably, while late-infantile MLD progresses rapidly, later-onset forms (juvenile and adult variants) exhibit slower disease progression, suggesting a potential therapeutic window even after symptom manifestation. Therefore, it is critical to assess the safety and efficacy of HSCGT in symptomatic patients.
On June 25, 2024, a collaborative research team led by Professor Qizhou Lian (Faculty of Synthetic Biology, Shenzhen University of Advanced Technology/ Institute of Synthetic Biology (iSynBio), Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences), together with partners from Guangzhou Women and Children’s Medical Center (affiliated with Guangzhou Medical University), Shenzhen Second People’s Hospital, Shenzhen Children’s Hospital, and The University of Hong Kong, published a research article in Protein & Cell titled: “Lentivirus-modified hematopoietic stem cell gene therapy for advanced symptomatic juvenile metachromatic leukodystrophy: A long-term follow-up pilot study”. This study reports nearly a decade of follow-up data on the safety and efficacy of autologous HSCGT in a symptomatic juvenile-onset MLD patient.
Back in 2014, Prof. Lian’s team pioneered the first application of HSCGT for a symptomatic juvenile-onset MLD patient.in Asia They conducted a multi-center, single-arm, open-label clinical trial (NCT02559830), evaluating long-term safety (via short- and long-term adverse event monitoring) and clinical efficacy (assessed through ARSA enzyme activity , MRI scoring, and neurological function assessments).
To date, six patients have been screened, with enrollment ongoing. The published findings focus on the first three enrolled patients, with follow-up durations of 4.5, 6.9, and 9.6 years, respectively. Safety assessments revealed that early adverse events (primarily chemotherapy-induced neutropenia) resolved with standard care, while no HSCGT-related complications emerged during long-term monitoring. Genome integration analysis confirmed stable engraftment of functional ARSA in hematopoietic and peripheral blood mononuclear cells. The research team placed particular emphasis on evaluating the risk of mutation or oncogene activation due to random insertion by lentiviral vectors. Whole-genome sequencing and PCR analysis identified 890 insertion sites, which were ebroadly distributed without chromosomal clustering. Most insertions occurred in intronic regions, with only 12 in exons, promoters, or 3’UTRs—and none near oncogenes, mitigating concerns over insertional mutagenesis.
The efficacy analysis revealed a significant increase in serum ARSA enzyme activity post-HSCGT, sustaining levels exceeding the normal physiological range. Compared to untreated controls, serial MRI assessments demonstrated marked improvement with stable scores over time, indicating attenuated neurodegenerative progression. Neurological function improved significantly, reflected by higher GMFC-MLD and FIM scores post-treatment, demonstrating HSCGT’s capacity to restore independent daily living skills. Collectively, these findings establish that HSCGT elevates ARSA activity, decelerates neurodegeneration, and enhances functional independence in symptomatic juvenile MLD patients, while maintaining a favorable safety profile. This study represents a pivotal advancement in treating inherited leukodystrophies and accelerates China’s leadership in cell and gene therapy translation.
Prof. Qizhou Lian from the Faculty of Synthetic Biology at Shenzhen University of Advanced Technology / Institute of Synthetic Biology (iSynBio), Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, served as corresponding author, with co-first authors Dr. Zhao Zhang (Guangzhou Women and Children’s Medical Center / The University of Hong Kong, now at Sun Yat-sen University), Prof. Hua Jiang(Guangzhou Women and Children’s Medical Center), and Dr. Xiaoya Zhou(SIAT/University of Macau), Prof. Yun Cai and Dr. Li Huang (Shenzhen Second People’s Hospital), and Prof. Sixi Liu(Shenzhen Children’s Hospita). Key contributors included Prof. Hongsheng Liu(Guangzhou Women and Children’s Medical Center), and Professors Jiacai Zhuo, Xin Du, and Ming Li(Shenzhen Second People’s Hospital). By demonstrating durable safety and efficacy in symptomatic juvenile MLD, this decade-long effort opens new therapeutic avenues for patients typically diagnosed too late for existing interventions.
